
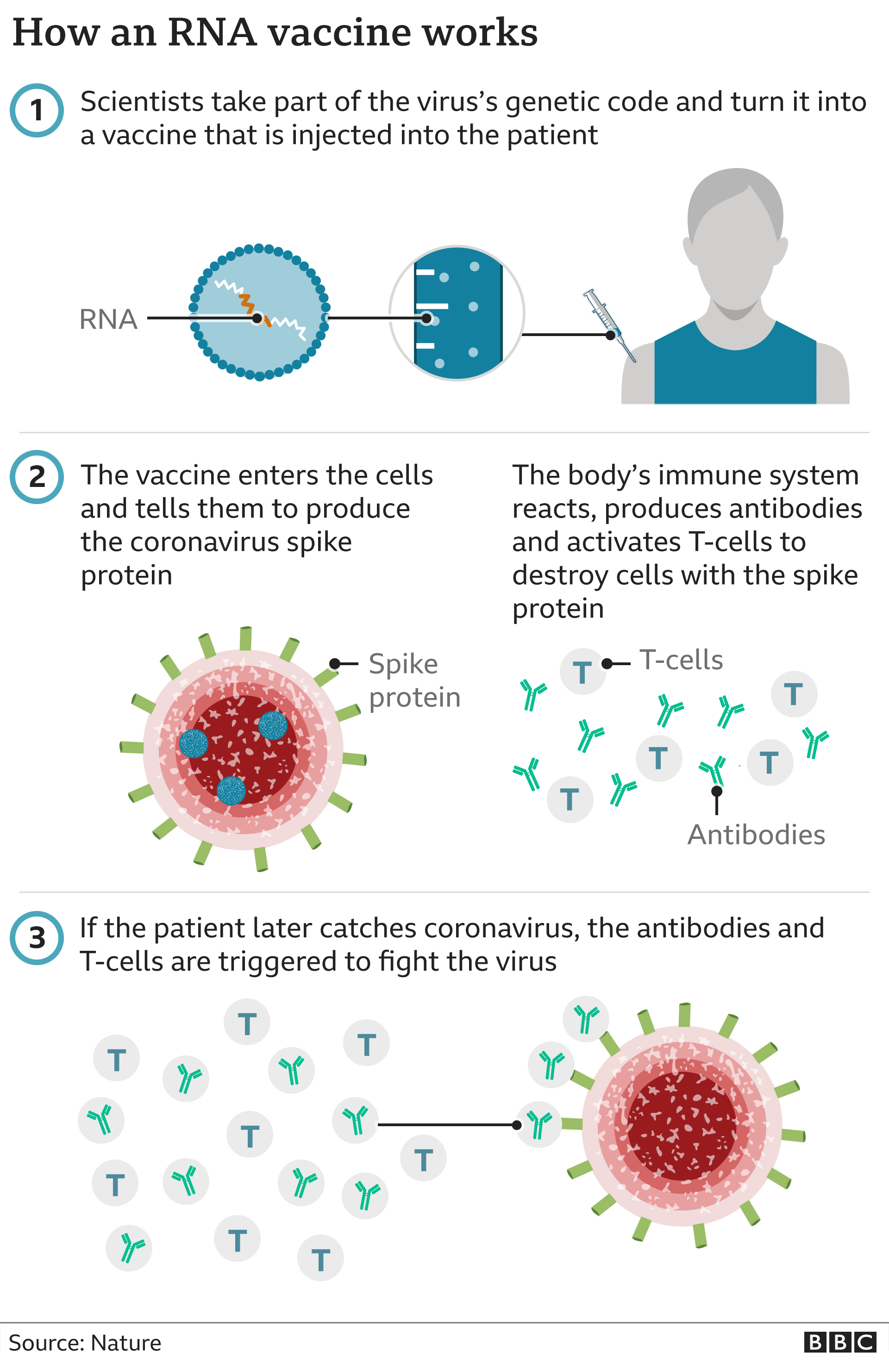
The initial 2 doses are administered 21 days apart followed by a third dose administered at least 8 weeks after the second dose.ĭose volume depends on which series of the vaccine is administered. The dosing for 6 months to less than 5 years is a 3-dose primary series of 3 micrograms each of the vaccine. The dosing schedule approved by Health Canada is to give 2 doses (30 micrograms each for ages 12 and older or 10 micrograms each for ages 5 to 11) 21 days apart, based on evidence from clinical trials. This means that the booster is expected to offer protection against this variant. The Pfizer-BioNTech Comirnaty ® Original and Omicron BA.1, bivalent COVID-19 vaccine was shown to increase the immune response against the Omicron BA.1 variant.This means that the primary series and booster is expected to offer protection against these variants. The Pfizer-BioNTech Comirnaty ® Original and Omicron BA.4/BA.5, bivalent COVID-19 vaccine was shown to increase the immune response against the Omicron BA.4/BA.5 variants.The data determined that the immune response to the vaccine for both age groups was comparable to the immune response of the older participants.
90.7% effective for those 5 to 11 years oldĮffectiveness data supporting the authorization in children ages 6 months to under 5 years are based on a comparison of immune responses in this age group to individuals ages 16 to 25 years.100% effective for those 12 to 15 years old.
POSSIBLE SIDE EFFECTS OF COVID VACCINE FDA TRIAL
The Pfizer-BioNTech Comirnaty ® Original and Omicron BA.1, bivalent COVID-19 vaccine is approved as a booster for people who are 12 years of age and older.

Pfizer-BioNTech Comirnaty ® Original and Omicron BA.1, bivalent COVID-19 vaccine The Pfizer-BioNTech Comirnaty ® Original and Omicron BA.4/BA.5, bivalent COVID-19 vaccine is approved as a primary series and a booster for people who are 5 years of age and older. Pfizer-BioNTech Comirnaty ® Original and Omicron BA.4/BA.5, bivalent COVID-19 vaccine This vaccine is also approved as a booster for people age 5 to 11 years as well as 16 years and older. Its safety and effectiveness in people younger than 6 months of age have not yet been established. The vaccine is approved for people who are 6 months of age and older. Pfizer-BioNTech Comirnaty ® COVID-19 vaccine


 0 kommentar(er)
0 kommentar(er)
